What Does the IRB Review?, Research
Por um escritor misterioso
Last updated 20 setembro 2024

Below are the elements the IRB looks for when reviewing research. Federal regulations 45 CFR 46.111 and 21 CFR 56.111 outline the requirements for approval of non-exempt human subjects research. To obtain IRB approval, the IRB must have enough information to determine the criteria in each of the sections below are satisfied.

Confluence Mobile - Confluence

PDF] The purpose, composition, and function of an institutional review board: balancing priorities.

Requirements for Institutional Review Board (IRB) Review and HIPAA Waiver Documentation for RIF DUA Request Submissions
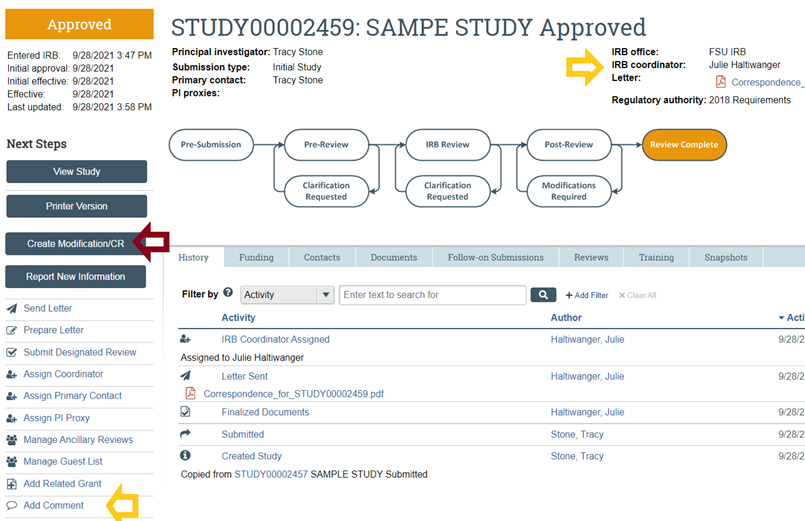
FAQs FSU Office of Research

Institutional Review Board - UA Little Rock
/research.png?n=4093)
Institutional Review Board Governors State University
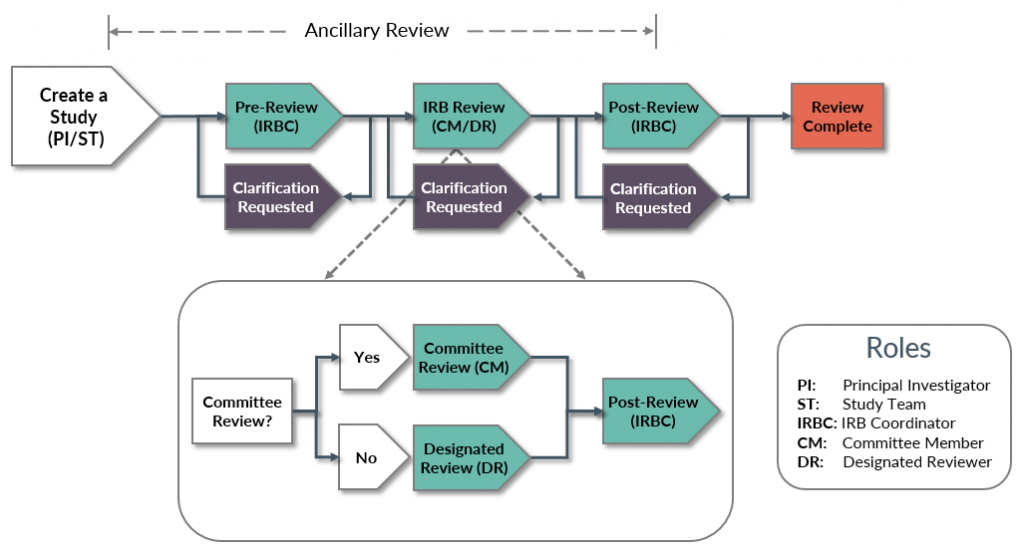
Overview of IRB Processes - UW Research
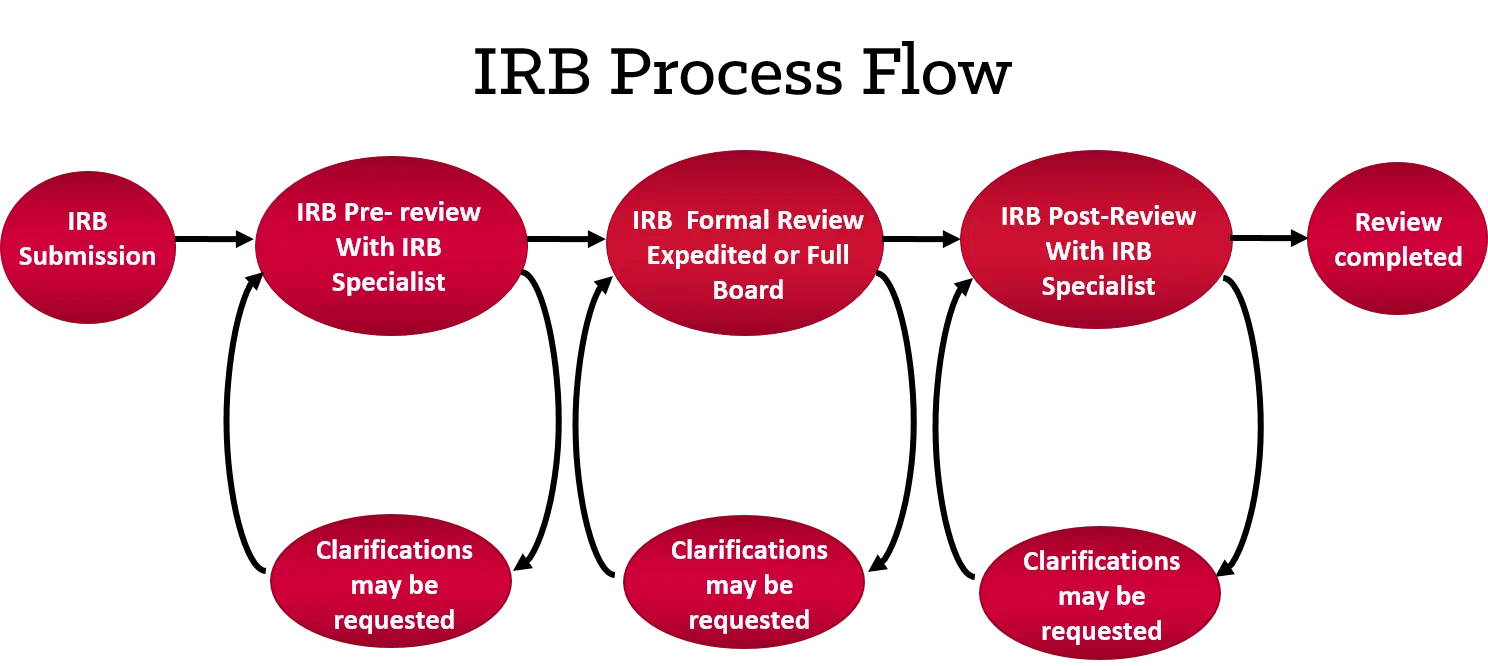
Institutional Review Board, Human Research Protection Program, University Hospitals, Cleveland, OH

IRB Review Process - Institutional Review Board - UA Little Rock

Overview of the IRB research review process.
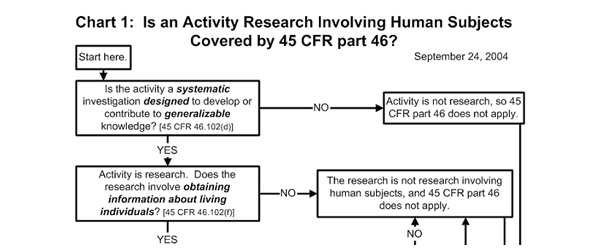
Going through the Institutional Review Board (IRB) Process For Informal Education Organizations
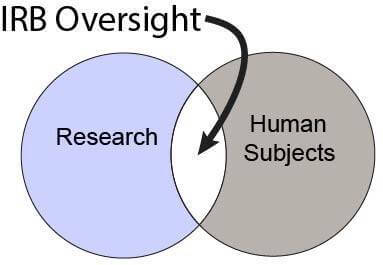
Is IRB Approval Required?
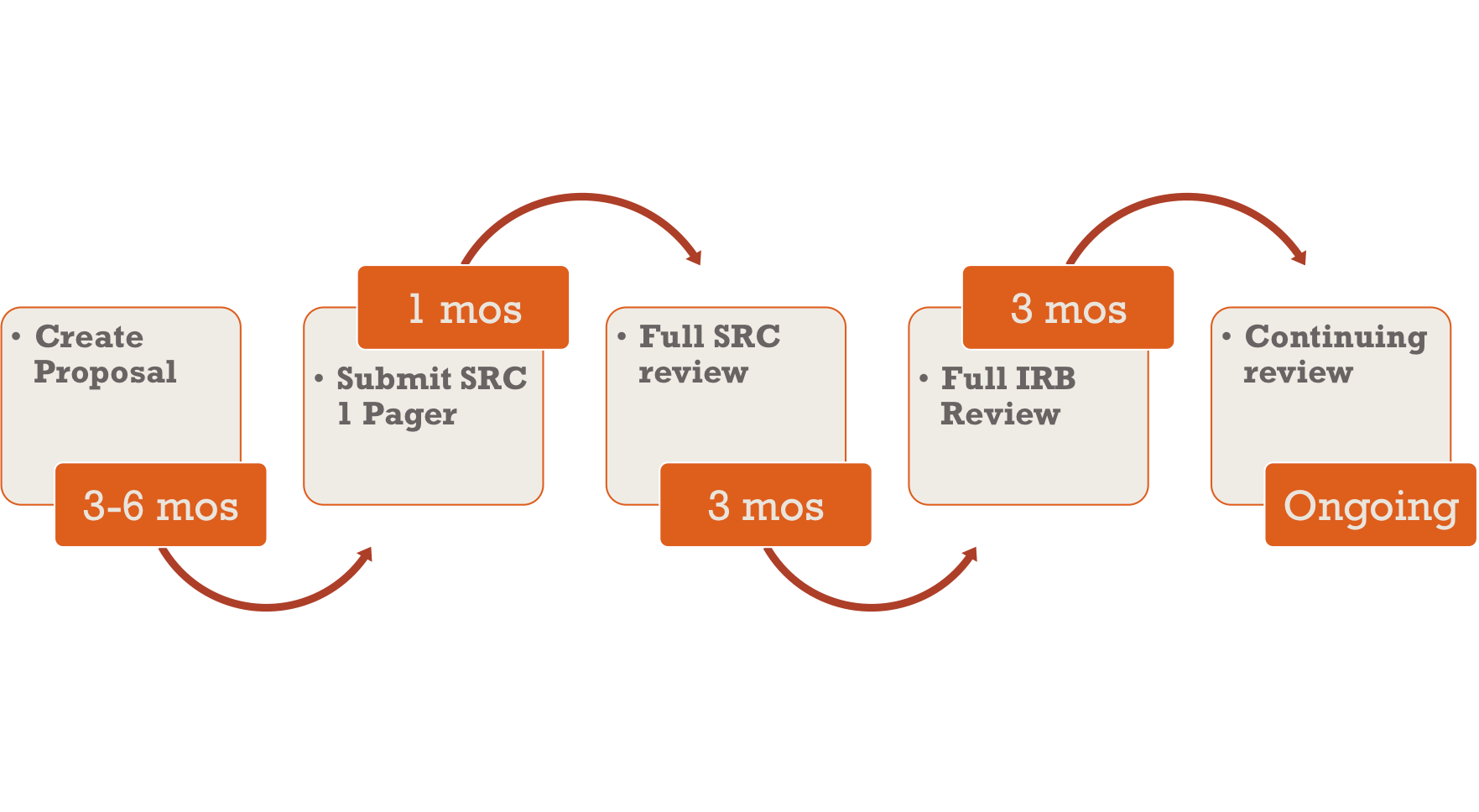
Scientific Review and IRB Submissions - National University of Natural Medicine
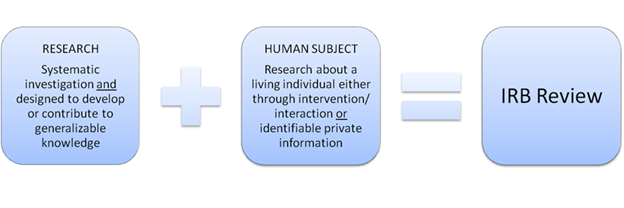
Human Subjects Research
Recomendado para você
-
 IRBSL – Instituto Rio Branco20 setembro 2024
IRBSL – Instituto Rio Branco20 setembro 2024 -
Instituto Rio Branco20 setembro 2024
-
 2019 – IRBSL20 setembro 2024
2019 – IRBSL20 setembro 2024 -
IRBSL20 setembro 2024
-
IRBSL - ♥poussin♥☺Sidi Lakhdar20 setembro 2024
-
 FUNDAMENTAL I e II – IRBSL20 setembro 2024
FUNDAMENTAL I e II – IRBSL20 setembro 2024 -
 适用于丰田本田日产车载腰靠座椅头颈枕抱枕四件套空调被logo定制-Taobao20 setembro 2024
适用于丰田本田日产车载腰靠座椅头颈枕抱枕四件套空调被logo定制-Taobao20 setembro 2024 -
 Yudia (@JoudiaBOUJDAINI) / X20 setembro 2024
Yudia (@JoudiaBOUJDAINI) / X20 setembro 2024 -
 Pew report: PA less restrictive on religion than Israel; Iran slightly worse20 setembro 2024
Pew report: PA less restrictive on religion than Israel; Iran slightly worse20 setembro 2024 -
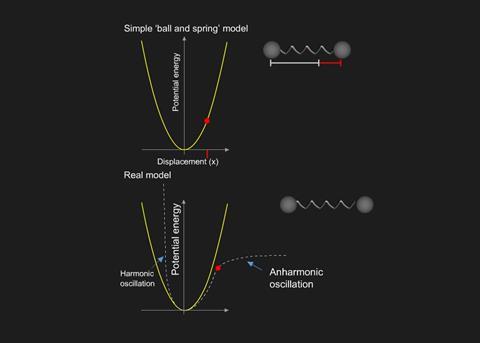 Infrared (IR) spectroscopy: Energy levels, Resource20 setembro 2024
Infrared (IR) spectroscopy: Energy levels, Resource20 setembro 2024
você pode gostar
-
Poki20 setembro 2024
-
 Kamado Tanjiro ( Demon Slayer ) Flaircygnus - Illustrations ART street20 setembro 2024
Kamado Tanjiro ( Demon Slayer ) Flaircygnus - Illustrations ART street20 setembro 2024 -
 Saint Seiya: Final Edition chegará ao Brasil em 202320 setembro 2024
Saint Seiya: Final Edition chegará ao Brasil em 202320 setembro 2024 -
 How to Download Videos Through (Mobile)20 setembro 2024
How to Download Videos Through (Mobile)20 setembro 2024 -
 Cinema: notícias e estreias - Cultura - Último Segundo - iG20 setembro 2024
Cinema: notícias e estreias - Cultura - Último Segundo - iG20 setembro 2024 -
 Diablo Immortal Known Issues, Hotfixes, and Patch Notes for PC20 setembro 2024
Diablo Immortal Known Issues, Hotfixes, and Patch Notes for PC20 setembro 2024 -
 Jogo Fifa Para Pc com Preços Incríveis no Shoptime20 setembro 2024
Jogo Fifa Para Pc com Preços Incríveis no Shoptime20 setembro 2024 -
 BatmAndrew.art on X: Dragonball GT Squad v.220 setembro 2024
BatmAndrew.art on X: Dragonball GT Squad v.220 setembro 2024 -
 Cabelo emo : r/HUEstation20 setembro 2024
Cabelo emo : r/HUEstation20 setembro 2024 -
 What Lex Fridman looks for in a relationship20 setembro 2024
What Lex Fridman looks for in a relationship20 setembro 2024



