Relative Uptake, Metabolism, and β-Receptor Binding of (1R,2S)-4
Por um escritor misterioso
Last updated 19 setembro 2024
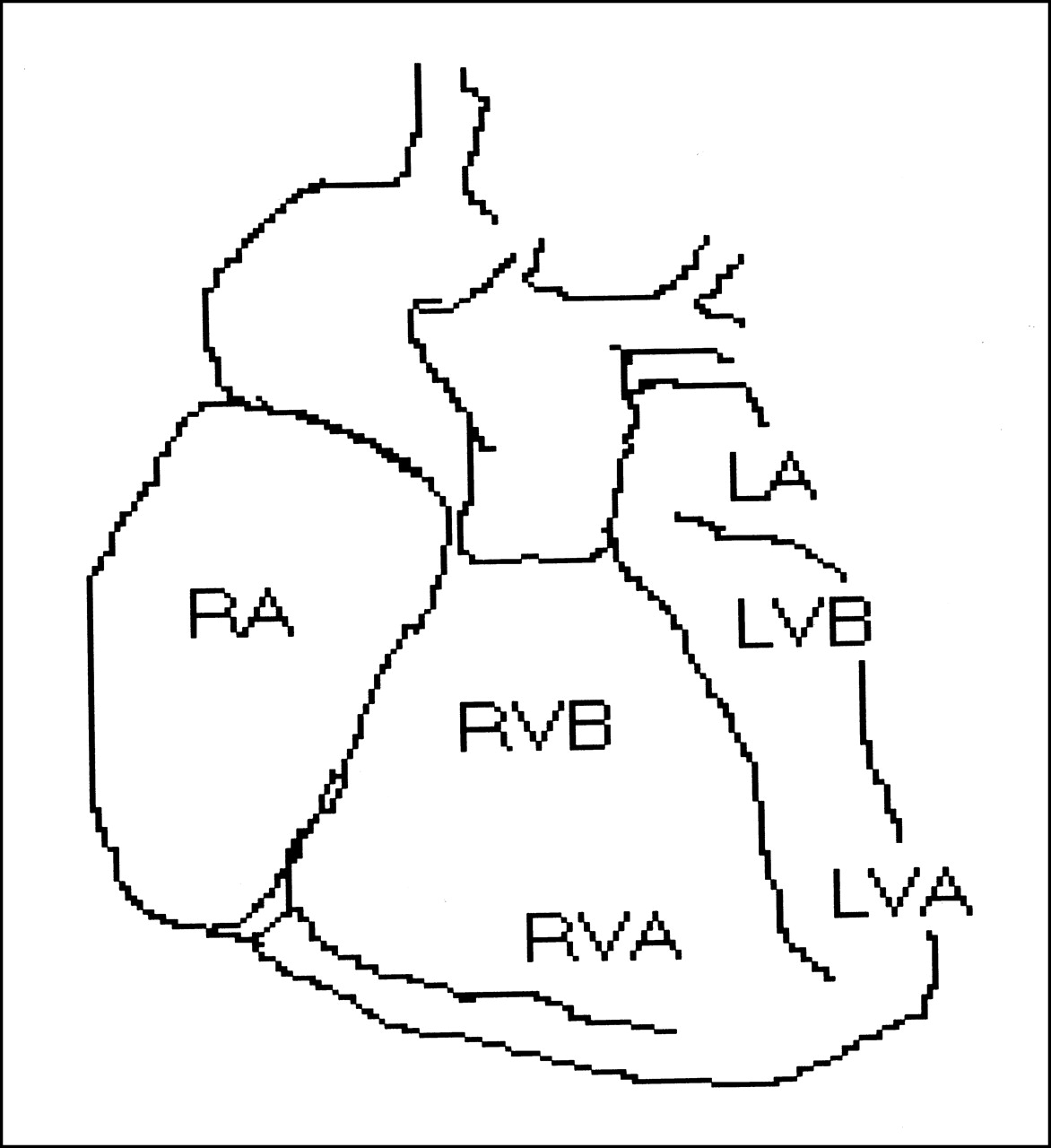
The objective of the study was to compare relative uptake, metabolism, and β-receptor affinity of the new positron-emitting uptake-1 tracer (1 R ,2 S )-4-18F-fluorometaraminol (4-FM) with those of the SPECT pharmaceutical meta-123I-iodobenzylguanidine (MIBG) in Wistar Kyoto (WKY) rats and spontaneously hypertensive (SHR) rats. Methods: No-carrier-added 4-18F-FM was applied to SHR and WKY rats in vivo and to retrogradely perfused hearts in vitro. Cardiac and extracardiac distribution was assessed, and metabolite formation was determined by thin-layer chromatography. The in vivo experiments were repeated with no-carrier-added 123I-MIBG. By means of autoradiography, the β-receptor affinity of 4-FM was compared with that of MIBG and propranolol (10 μmol/L) through displacement of 125I-iodocyanopindolol (1.5 pmol/L) in slices of heart and spleen. Results: Cardiomyopathic hearts showed heterogeneous 4-18F-FM uptake with gradients up to 3.6 in vivo and in vitro between different regions of the heart. Control hearts showed such gradients in 4-18F-FM uptake only in vitro. 123I-MIBG exhibited a less heterogeneous in vivo distribution in SHR hearts. Extracardiac differences between WKY and SHR were found for uptake of 4-18F-FM in the spleen (63.3% ± 4% vs. 38.8% ± 5.7% of cardiac activity) and for renal uptake of 123I-MIBG (373% ± 27% vs. 81.4% ± 17% of cardiac activity). Metabolites of 4-18F-FM were found only in the liver and those of 123I-MIBG were found in the liver and kidney with a nearly equal relative fraction in both types of animals of about 20%, 60%, and 30%, respectively. 4-FM suppressed cardiac-specific β-receptor binding of 125I-iodocyanopindolol in heart and spleen of both types of animals significantly, whereas MIBG had almost no effect. Conclusion: The more heterogeneous cardiac distribution of 4-18F-FM suggests that it reflects alterations in uptake-1 better than 123I-MIBG in addition to the possibility of quantification and higher spatial resolution by PET compared with SPECT. Altered biotransformation in cardiomyopathic diseases may also impair the evaluation of 123I-MIBG-SPECT data. The β-receptor binding of 4-18F-FM must be further elucidated.

Hexokinase 1 cellular localization regulates the metabolic fate of glucose - ScienceDirect
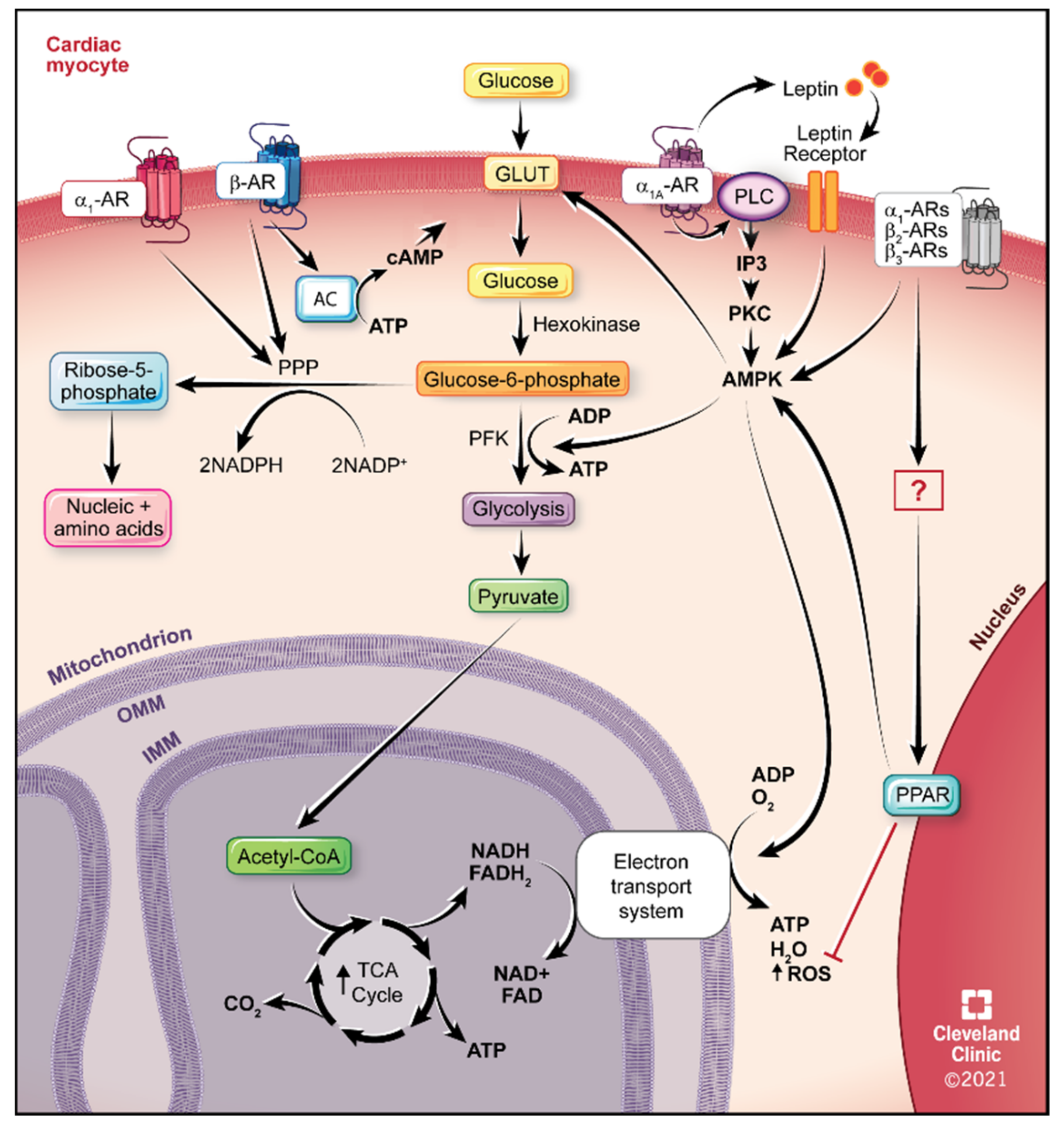
IJMS, Free Full-Text
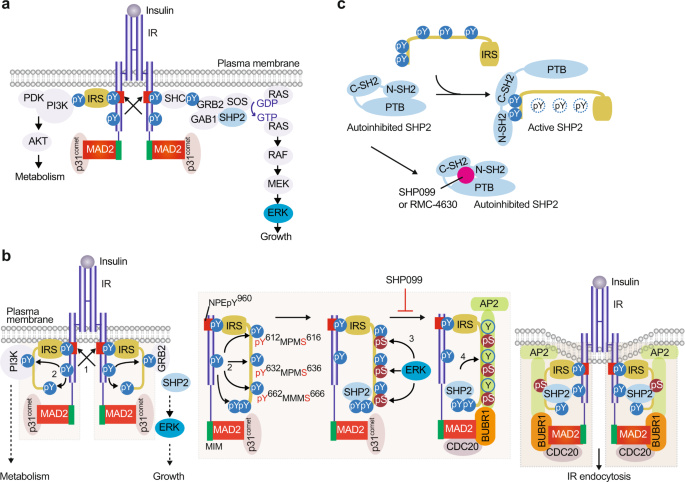
Insulin receptor endocytosis in the pathophysiology of insulin resistance

Full article: Pathogenicity and virulence of West Nile virus revisited eight decades after its first isolation

Metabolic Reprogramming Induces Germinal Center B Cell Differentiation through Bcl6 Locus Remodeling - ScienceDirect
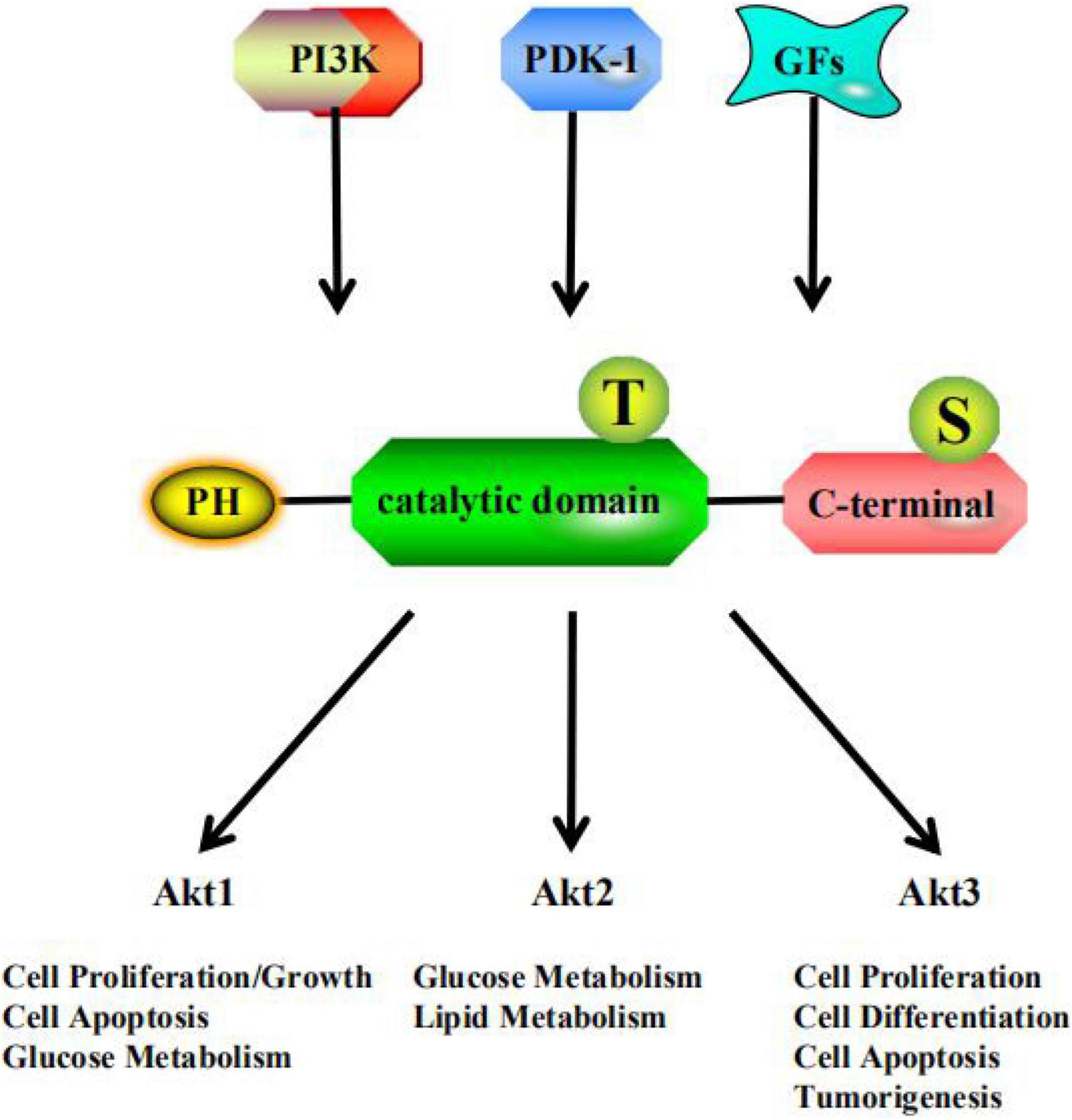
Frontiers Akt: A Potential Drug Target for Metabolic Syndrome
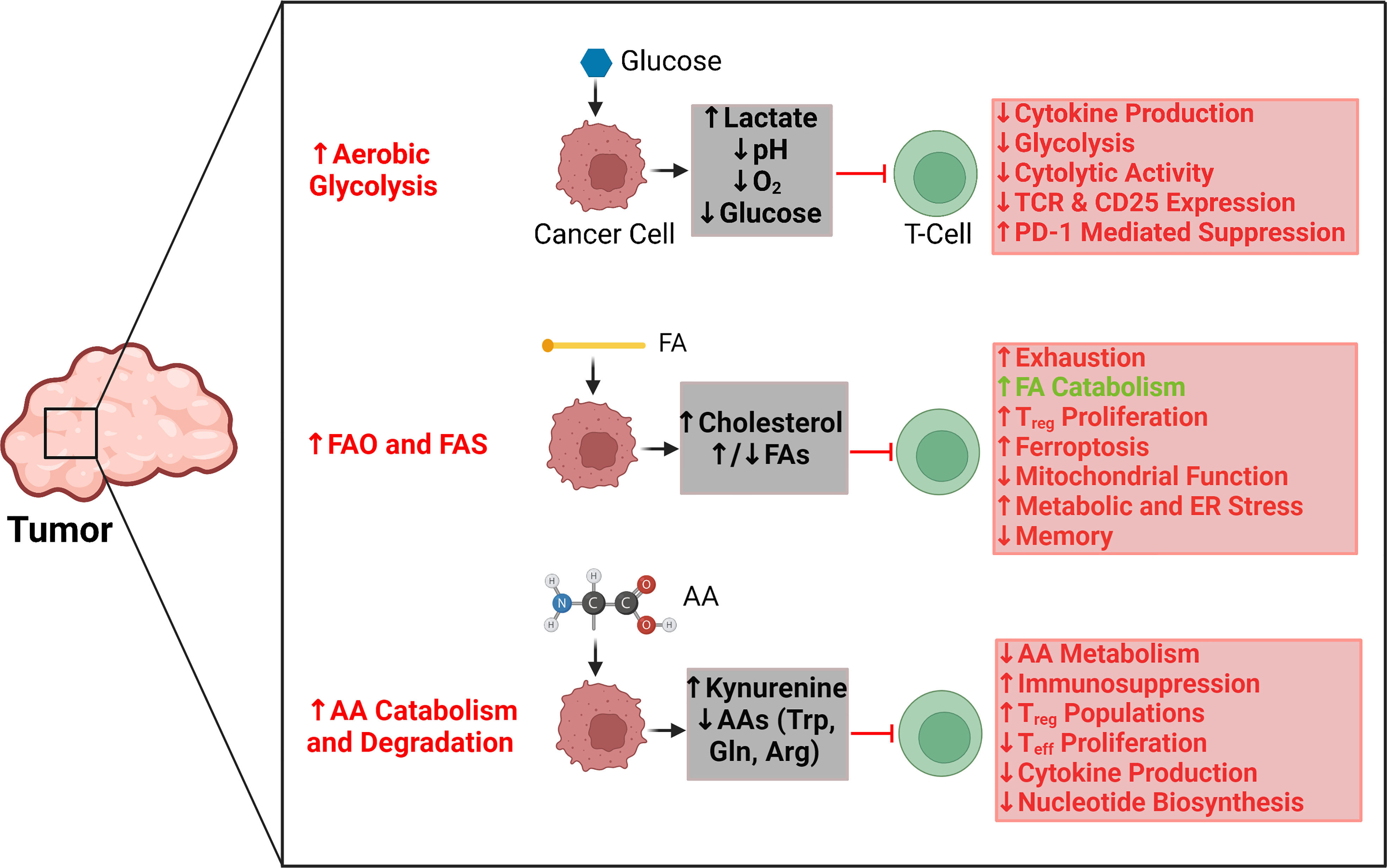
Frontiers The role of tumor metabolism in modulating T-Cell activity and in optimizing immunotherapy

Glucagon-like Peptide-1 (GLP-1) Analogs: Recent Advances, New Possibilities, and Therapeutic Implications

Hormonal regulation of glucose metabolism by insulin. Insulin is

Calciprotein Particles Induce Endothelial Dysfunction by Impairing Endothelial Nitric Oxide Metabolism

Structural perspective of class B1 GPCR signaling: Trends in Pharmacological Sciences

Disposition of Nirmatrelvir, an Orally Bioavailable Inhibitor of SARS-CoV-2 3C-Like Protease, across Animals and Humans
Recomendado para você
-
 Vivo Reserva Merlot19 setembro 2024
Vivo Reserva Merlot19 setembro 2024 -
 Números 14:28 RVA - Diles: Vivo yo, dice Jehová, que según habéis19 setembro 2024
Números 14:28 RVA - Diles: Vivo yo, dice Jehová, que según habéis19 setembro 2024 -
Tropicabana RVA, Richmond VA19 setembro 2024
-
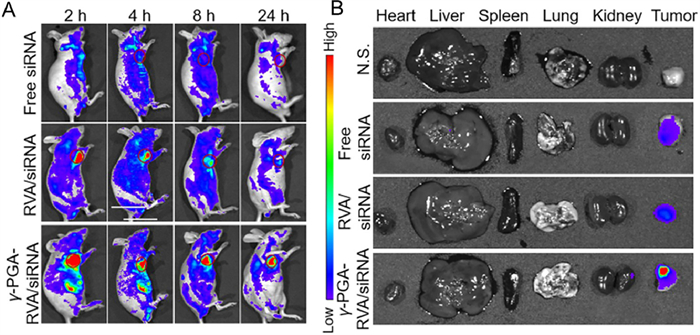 Integrated and dual-responsive lipopeptide nanovector with19 setembro 2024
Integrated and dual-responsive lipopeptide nanovector with19 setembro 2024 -
 Qué Pasa? Festival is Rescheduled Due to Weather - RVAHub19 setembro 2024
Qué Pasa? Festival is Rescheduled Due to Weather - RVAHub19 setembro 2024 -
MR PULPO Richmond VA19 setembro 2024
-
 Momentum Voya E+ 1 - Pedal Power RVA19 setembro 2024
Momentum Voya E+ 1 - Pedal Power RVA19 setembro 2024 -
 Imitation of RVA pasting measurement protocol in an oscillatory19 setembro 2024
Imitation of RVA pasting measurement protocol in an oscillatory19 setembro 2024 -
 RVA em direto Rádio Online Grátis19 setembro 2024
RVA em direto Rádio Online Grátis19 setembro 2024 -
 GazetaWeb - Marinha constata semelhança entre bola de fogo e19 setembro 2024
GazetaWeb - Marinha constata semelhança entre bola de fogo e19 setembro 2024
você pode gostar
-
Mangás Brasil - Chegando ao Japão Grand Blue #14 Grand Blue também conhecido como Grand Blue Dreaming, é uma série de mangá japonesa escrita por Kenji Inoue e ilustrada por Kimitake Yoshioka.19 setembro 2024
-
 roronoa zoro símbolos bandeira 1 peça 21857991 Vetor no Vecteezy19 setembro 2024
roronoa zoro símbolos bandeira 1 peça 21857991 Vetor no Vecteezy19 setembro 2024 -
![Funkin' in the Backrooms [Friday Night Funkin'] [Mods]](https://images.gamebanana.com/img/ss/mods/64ea261a64b9e.jpg) Funkin' in the Backrooms [Friday Night Funkin'] [Mods]19 setembro 2024
Funkin' in the Backrooms [Friday Night Funkin'] [Mods]19 setembro 2024 -
 Mahou Shoujo of the End #10 - Vol. 10 (Issue)19 setembro 2024
Mahou Shoujo of the End #10 - Vol. 10 (Issue)19 setembro 2024 -
 Galar Zapdos by mythicalmunchkin on DeviantArt19 setembro 2024
Galar Zapdos by mythicalmunchkin on DeviantArt19 setembro 2024 -
 Transformations of Ichigo - Vasto Lorde form by RomaniacC on DeviantArt19 setembro 2024
Transformations of Ichigo - Vasto Lorde form by RomaniacC on DeviantArt19 setembro 2024 -
 West Ham playing dangerous game as the Moyes project stops working, West Ham United19 setembro 2024
West Ham playing dangerous game as the Moyes project stops working, West Ham United19 setembro 2024 -
 For XBOX 360 controllers case cover Vinyl Decal Skin sticker-Japanese anime19 setembro 2024
For XBOX 360 controllers case cover Vinyl Decal Skin sticker-Japanese anime19 setembro 2024 -
 MF en inglés: Significado y uso - UDOE19 setembro 2024
MF en inglés: Significado y uso - UDOE19 setembro 2024 -
 Boruto - Naruto sofre um destino pior que a morte no mangá!19 setembro 2024
Boruto - Naruto sofre um destino pior que a morte no mangá!19 setembro 2024


