Assembly of recombinant tau into filaments identical to those of
Por um escritor misterioso
Last updated 19 setembro 2024
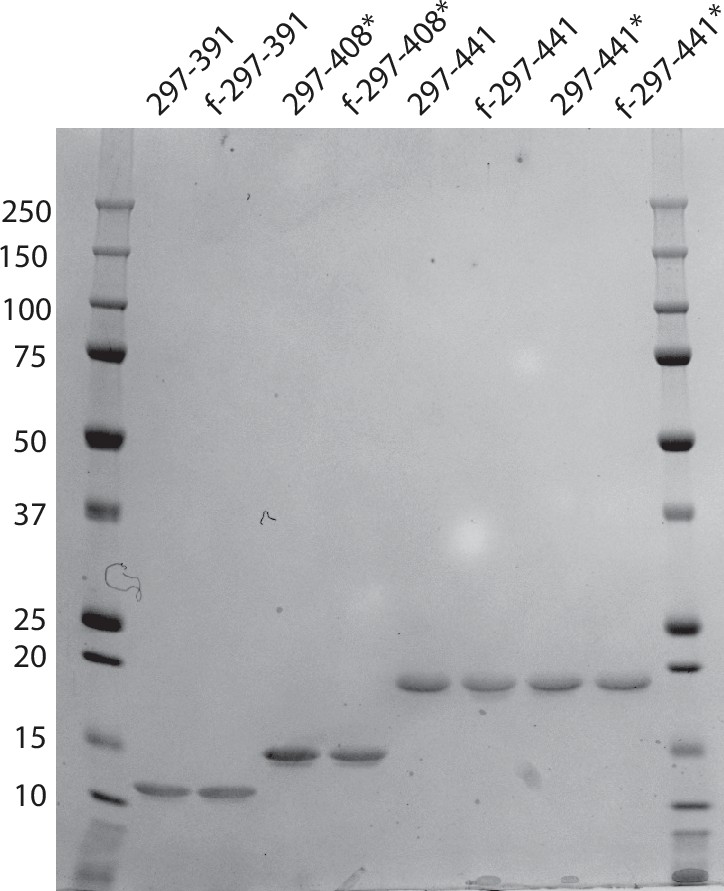
Many neurodegenerative diseases, including Alzheimer’s disease, the most common form of dementia, are characterised by knotted clumps of a protein called tau. In these diseases, tau misfolds, stacks together and forms abnormal filaments, which have a structured core and fuzzy coat. These sticky, misfolded proteins are thought to be toxic to brain cells, the loss of which ultimately causes problems with how people move, think, feel or behave. Reconstructing the shape of tau filaments using an atomic-level imaging technique called electron cryo-microscopy, or cryo-EM, researchers have found distinct types of tau filaments present in certain diseases. In Alzheimer’s disease, for example, a mixture of paired helical and straight filaments is found. Different tau filaments are seen again in chronic traumatic encephalopathy (CTE), a condition associated with repetitive brain trauma. It remains unclear, however, how tau folds into these distinct shapes and under what conditions it forms certain types of filaments. The role that distinct tau folds play in different diseases is also poorly understood. This is largely because researchers making tau proteins in the lab have yet to replicate the exact structure of tau filaments found in diseased brain tissue. Lövestam et al. describe the conditions for making tau filaments in the lab identical to those isolated from the brains of people who died from Alzheimer’s disease and CTE. Lövestam et al. instructed bacteria to make tau protein, optimised filament assembly conditions, including shaking time and speed, and found that bona fide filaments formed from shortened versions of tau. On cryo-EM imaging, the lab-produced filaments had the same left-handed twist and helical symmetry as filaments characteristic of Alzheimer’s disease. Adding salts, however, changed the shape of tau filaments. In the presence of sodium chloride, otherwise known as kitchen salt, tau formed filaments with a filled cavity at the core, identical to tau filaments observed in CTE. Again, this structure was confirmed on cryo-EM imaging. Being able to make tau filaments identical to those found in human tauopathies will allow scientists to study how these filaments form and elucidate what role they play in disease. Ultimately, a better understanding of tau filament formation could lead to improved diagnostics and treatments for neurodegenerative diseases involving tau.
Laboratory-based methods are presented that produce filamentous tau aggregates with the same structures as those observed in neurodegenerative disease.
Laboratory-based methods are presented that produce filamentous tau aggregates with the same structures as those observed in neurodegenerative disease.
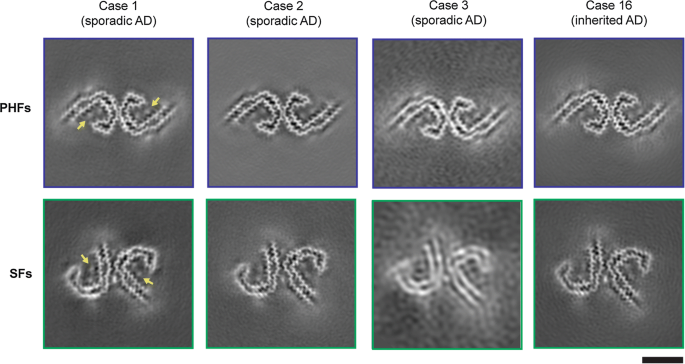
Tau filaments from multiple cases of sporadic and inherited Alzheimer's disease adopt a common fold

Immuno-EM of tau filaments from the frontal cortex of sporadic and
Truncation of tau at Asp 421 increases the rate and extent of tau

Conformation Determines the Seeding Potencies of Native and Recombinant Tau Aggregates - ScienceDirect
RCSB PDB - 7QK2: In vitro assembled 300-391 tau filaments in PBS (36a)
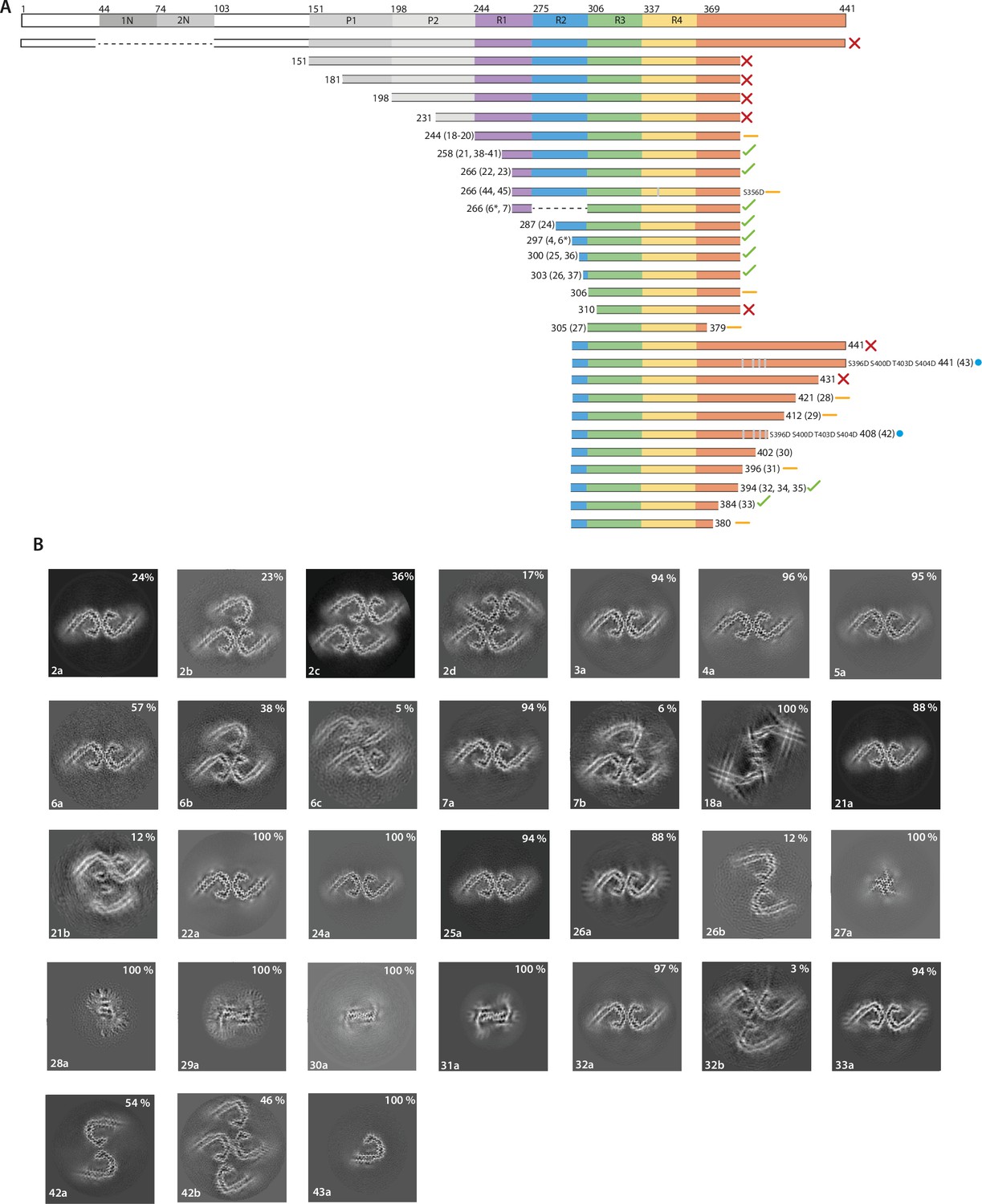
Assembly of recombinant tau into filaments identical to those of Alzheimer's disease and chronic traumatic encephalopathy
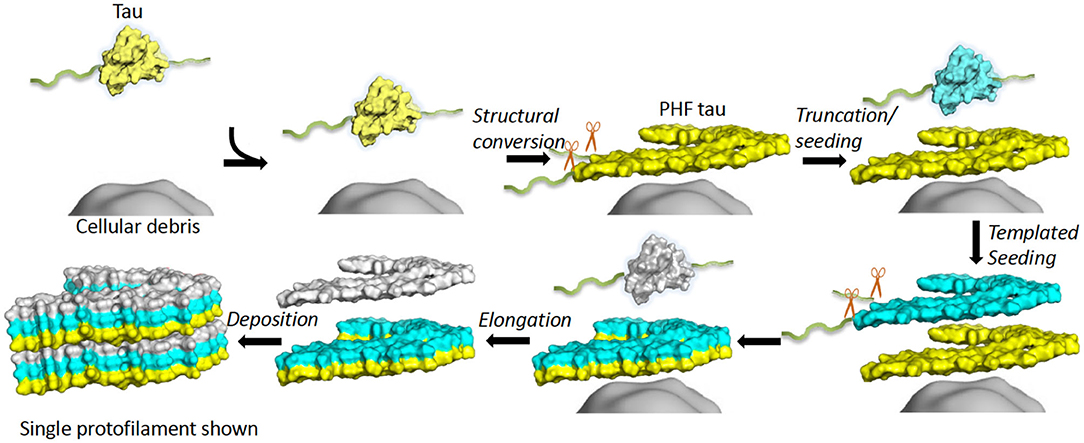
Frontiers Tau Filament Self-Assembly and Structure: Tau as a Therapeutic Target
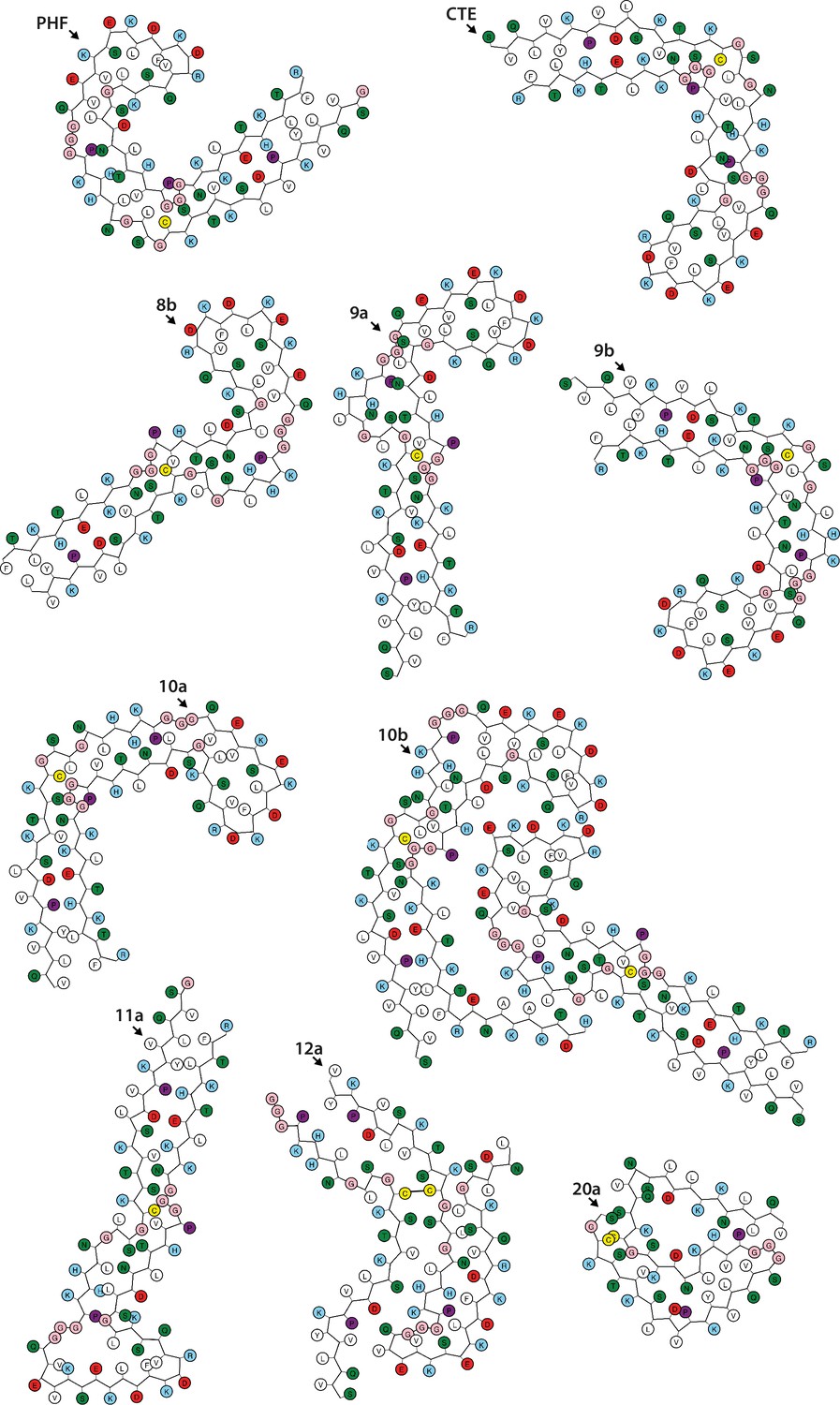
Assembly of recombinant tau into filaments identical to those of Alzheimer's disease and chronic traumatic encephalopathy

Subtle change of fibrillation condition leads to substantial alteration of recombinant Tau fibril structure - ScienceDirect

Chemical Synthesis of Microtubule-Associated Protein Tau
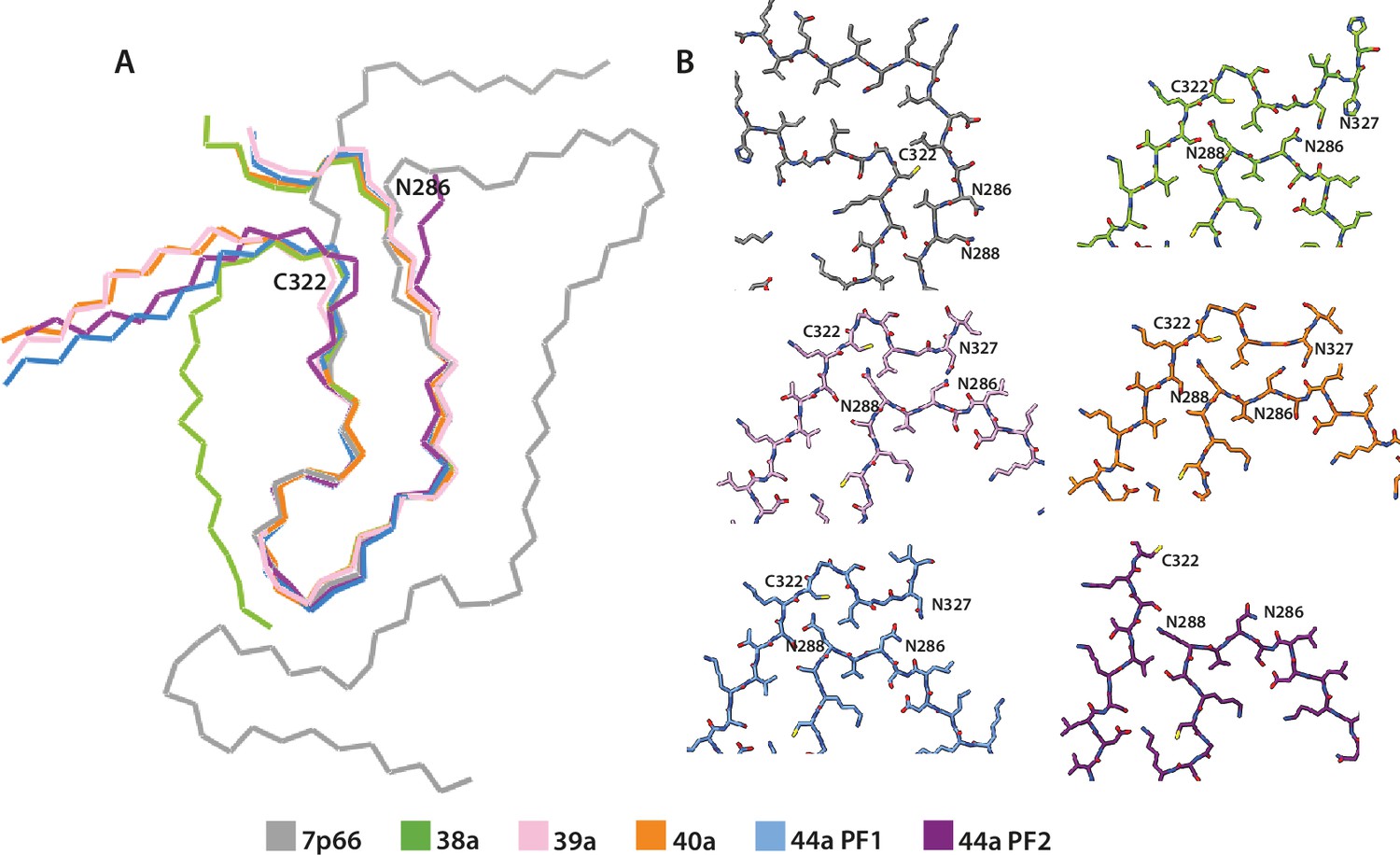
Assembly of recombinant tau into filaments identical to those of Alzheimer's disease and chronic traumatic encephalopathy
Recomendado para você
-
 حل Brain Test من المرحلة 280 إلى المرحلة 30019 setembro 2024
حل Brain Test من المرحلة 280 إلى المرحلة 30019 setembro 2024 -
Brain test level 297 - Siapa yang menumpahkan susu? #jawaban19 setembro 2024
-
 Observation Brain Test: If you have 50/50 Vision Find the Number19 setembro 2024
Observation Brain Test: If you have 50/50 Vision Find the Number19 setembro 2024 -
 workflow4metabolomics (@workflow4metabo) / X19 setembro 2024
workflow4metabolomics (@workflow4metabo) / X19 setembro 2024 -
 Brain Test Level 297 We need a fire - Frenemy19 setembro 2024
Brain Test Level 297 We need a fire - Frenemy19 setembro 2024 -
 Brain Test: Tricky Words Level 296, 297, 298, 299, 300 Answers19 setembro 2024
Brain Test: Tricky Words Level 296, 297, 298, 299, 300 Answers19 setembro 2024 -
 Investors impatient for Alzheimer's cure - MarketWatch19 setembro 2024
Investors impatient for Alzheimer's cure - MarketWatch19 setembro 2024 -
 Should We Test for Diastolic Dysfunction? How and How Often19 setembro 2024
Should We Test for Diastolic Dysfunction? How and How Often19 setembro 2024 -
 BRAIN TEST, NIVEL 297 - ¡Sálvala! [GAMEPLAY19 setembro 2024
BRAIN TEST, NIVEL 297 - ¡Sálvala! [GAMEPLAY19 setembro 2024 -
Mitochondrial Disease19 setembro 2024
você pode gostar
-
 Linhas de ônibus terão reforço com o jogo do Brasil na Copa nesta19 setembro 2024
Linhas de ônibus terão reforço com o jogo do Brasil na Copa nesta19 setembro 2024 -
 Season 5 Stranger Things: Hawkins Will Fall In Like 2055 : r19 setembro 2024
Season 5 Stranger Things: Hawkins Will Fall In Like 2055 : r19 setembro 2024 -
 Como Desenhar o SONIC CORRENDO I Fácil19 setembro 2024
Como Desenhar o SONIC CORRENDO I Fácil19 setembro 2024 -
 Processo Seletivo 2022 do IFBA Jequié. Clique e saiba mais19 setembro 2024
Processo Seletivo 2022 do IFBA Jequié. Clique e saiba mais19 setembro 2024 -
 510 ideias de Carros e motos em 202319 setembro 2024
510 ideias de Carros e motos em 202319 setembro 2024 -
 GAME FPS PALING SERU DI ROBLOX 2019 #mancap - ROBLOX INDONESIA19 setembro 2024
GAME FPS PALING SERU DI ROBLOX 2019 #mancap - ROBLOX INDONESIA19 setembro 2024 -
 Westlife - I Wanna Grow Old With You (tradução)19 setembro 2024
Westlife - I Wanna Grow Old With You (tradução)19 setembro 2024 -
![Total Drama - Take The Crown [Gameplay] : r/Totaldrama](https://external-preview.redd.it/total-drama-take-the-crown-gameplay-v0-6S5S73wts63l8hgEDO3tiC56JaOUoEAetKosW90s-iI.jpg?auto=webp&s=0a360082b0b16075a857a5199e2c587fd577c233) Total Drama - Take The Crown [Gameplay] : r/Totaldrama19 setembro 2024
Total Drama - Take The Crown [Gameplay] : r/Totaldrama19 setembro 2024 -
![Cards] Hide of the Wild Guardian The Griffon's Saddlebag in 2023](https://i.pinimg.com/1200x/3a/c8/35/3ac835a813b0132484f584105f89bb30.jpg) Cards] Hide of the Wild Guardian The Griffon's Saddlebag in 202319 setembro 2024
Cards] Hide of the Wild Guardian The Griffon's Saddlebag in 202319 setembro 2024 -
![Call of Duty: Black Ops II [Standard edition] (Xbox 360)](https://m.media-amazon.com/images/W/MEDIAX_792452-T2/images/I/81tQkwAqmaL.jpg) Call of Duty: Black Ops II [Standard edition] (Xbox 360)19 setembro 2024
Call of Duty: Black Ops II [Standard edition] (Xbox 360)19 setembro 2024